Marvelous Tips About How To Write Nitric Acid

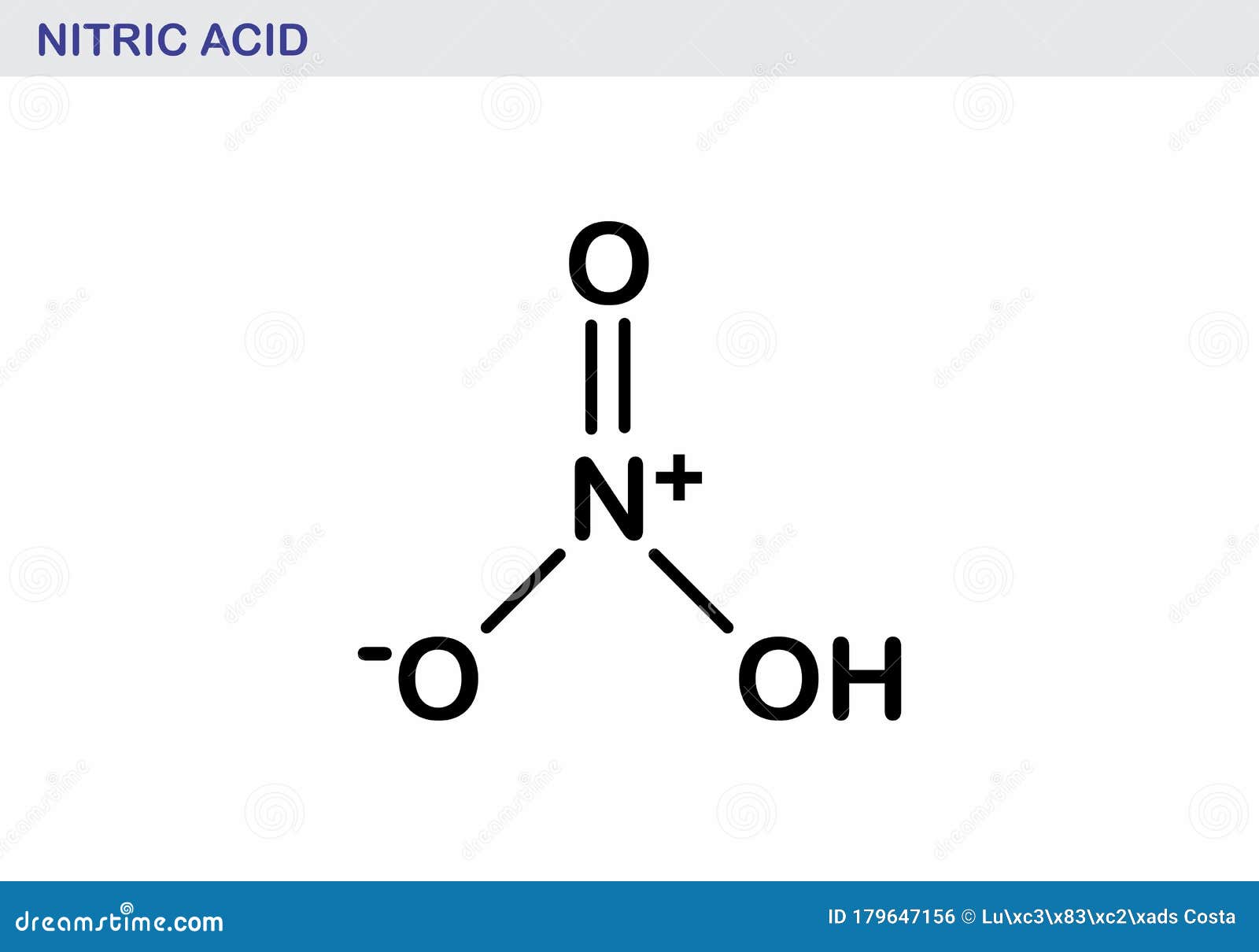
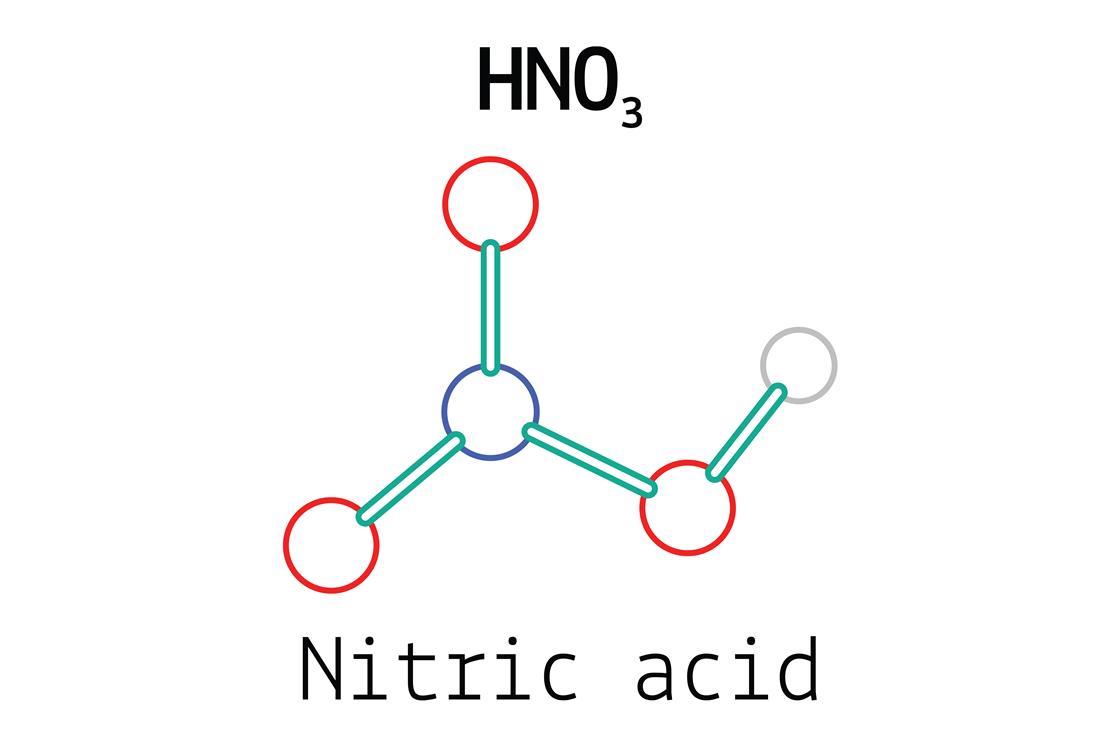
Structure of hno3 molecules.
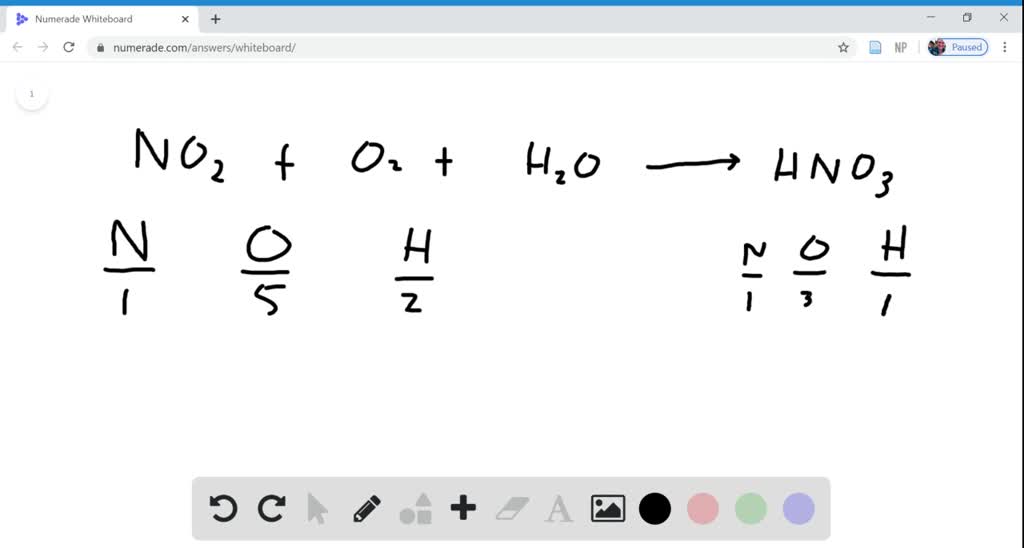
How to write nitric acid. Suggested mnemonic schemes indicate the reduction product in nitric acid. Behavior as an oxidizing agent will depend on the concentration of nitric acid. Writing formulas for acids;
Nitric acid is the inorganic compound with the formula hno3. Qi, in treatise on geochemistry (second edition), 2014. Nitric acid is a very common and heavily used chemical in the world and manufactured in large scale by global manufactures.
Nitric acid, (hno 3 ), colourless, fuming, and highly corrosive liquid (freezing point −42 °c [−44 °f], boiling point 83 °c [181 °f]) that is a common laboratory reagent. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into oxides of nitrogen. A spot test for gold has been in use for decades.
Other metals may react or dissolve in this acid, but gold will not. The hno3 lewis structure is best thought of as the no3 with an h attache. In this video we'll write the correct.
65k views 5 years ago. First, we balance the molecular equation. How to write complete ionic equations and net ionic equations.
0:00 / 1:28. A chemical formula represents the number of atoms of each element present in a molecule of the compound. Since acids produce \(\ce{h^+}\) cations upon dissolving in water, the \(\ce{h}\) of an acid is written first in the formula of an inorganic acid.
Get more practice writing formulas for compounds. It is a highly corrosive mineral acid. Spectroscopic studies have shown that in a gaseous state, nitric acid exists as a planar molecule with a bond angle and bond.
Dissolve 80 grams (2.8 oz) of nitrate salt in 50 millilitres (1.7 fl oz) of water. Because this acid contains a bromine atom, the name is hydrobromic acid. When the solution contains more than 86% hno3, it is referred to as fuming nitric ac…
Nitric acid (hno3) is one of the most widely used digestion reagents and the most. Most commercially available nitric acid has a concentration of 68% in water. The sample is first treated with nitric acid.
Nitric acid, hno 3, is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. The sample is first treated with nitric acid. Nitric acid is normally considered to be a.








.jpg)